Literature Review: COPDGene® 2019: Redefining the Diagnosis of Chronic Obstructive Pulmonary Disease
New Criteria Incorporates Quantitative CT Imaging in COPD Diagnosis
Introduction
In case you missed it, the COPDGene® investigators (112 pulmonary leaders) recently proposed a new methodology to diagnose and classify COPD, based on diagnostic information from over 10,000 smokers. This is big news!
Today’s diagnostic methods for COPD have been in practice since the 1950s[1][2]; spirometry, the primary instrument used for diagnosis, was invented in the mid-1800s[3]. Here’s a fun fact: The Liberty Bell was cracked in the same year the spirometer was invented. While it is a testament to the clinical impact of Dr. Hutchinson’s invention, surely there are technology and scientific advances in the last 170 years that could enhance and improve the standard of care for COPD patients. The proposal by the COPDGene investigators makes a strong case that CT imaging data is one of those
advancements that should be included in a COPD diagnosis.
Limitations of the Current Diagnostic Methodology
Spirometry has come a long way since 1846. It has been, and will continue to be, a valuable tool in assessing patients with or at risk of lung disease. It is effective, easy to operate, and well validated. However:
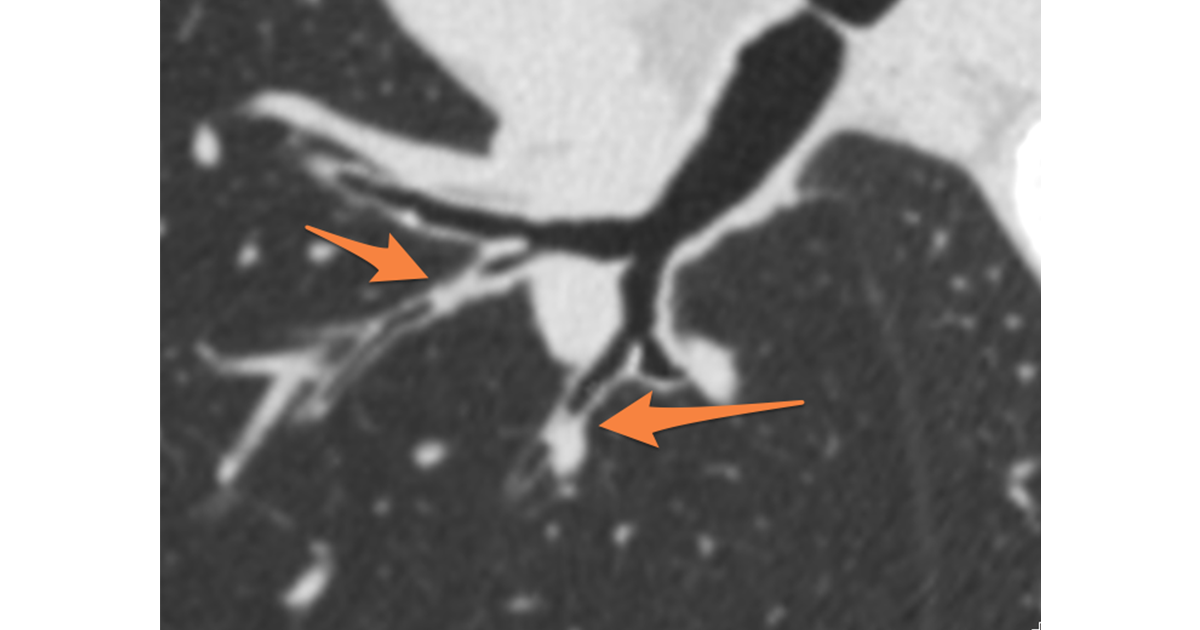
- Spirometry lacks the necessary sensitivity to pick up early disease in some patients. For example, several major studies have shown patient cohorts with normal spirometry measures, yet with significant anatomical airway and/or lung changes (wall thickening and/or emphysema), as visible by CT.[4], [5]
- Spirometry results are global lung measures and cannot provide localized, lobar level detail. Conversely, Quantitative CT (QCT) can provide both global and local measures.
- Spirometry fails to identify those with concurrent loss of FEV1 (Forced Expiratory Volume) and FVC (Forced Vital Capacity), since the FEV1/FVC ratio will remain normal despite presence/progression of disease.
The paper outlines three disease progression pathways for COPD, noting that two of the three are often missed by current diagnostic methodologies.
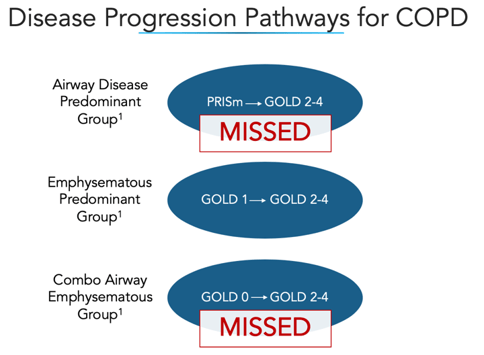
The COPDGene Diamond Methodology
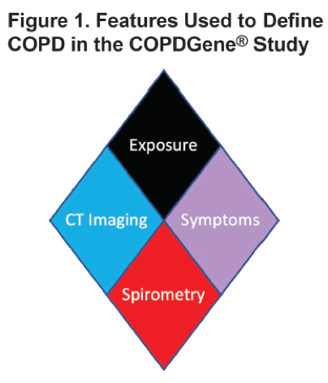
The authors suggest a 4-part criteria to assess a potential COPD patient:
- Exposure – Does the patient have 10 pack years of smoking history or more?
- Symptoms – Does the patient have an mMRC (modified medical research council) dyspnea score of ≥2 or chronic bronchitis?
- CT Structural Abnormality – Does the patient have a QCT (Quantitative Computed Tomography) showing ≥ 5% low density, a Pi10 value of ≥ 2.5mm or ≥15% gas trapping?
- Spirometry - Does the patient have an FEV1 < 80% predicted or an FEV1/FVC < 0.70?
These four criteria are depicted with a diamond
illustration, as shown below:
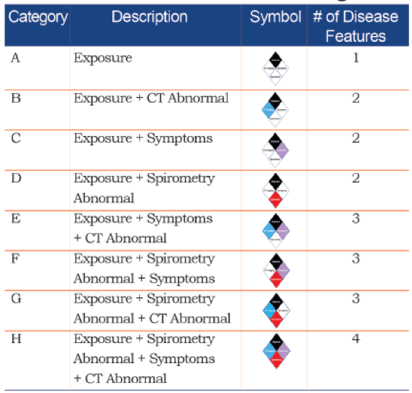
Based on the presence or absence of these criteria, 8 possible categories are possible, as shown below.
The paper then discussed how these 8 mutually exclusive categories
can be classified into “No COPD” (Category A), “Possible COPD” (B-D), “Probable
COPD” (E-G) or “Definite COPD.”
Category E, for example, includes patients with exposure, CT structural abnormalities, and symptoms. These patients would be classified as “probable COPD” despite normal spirometry results.
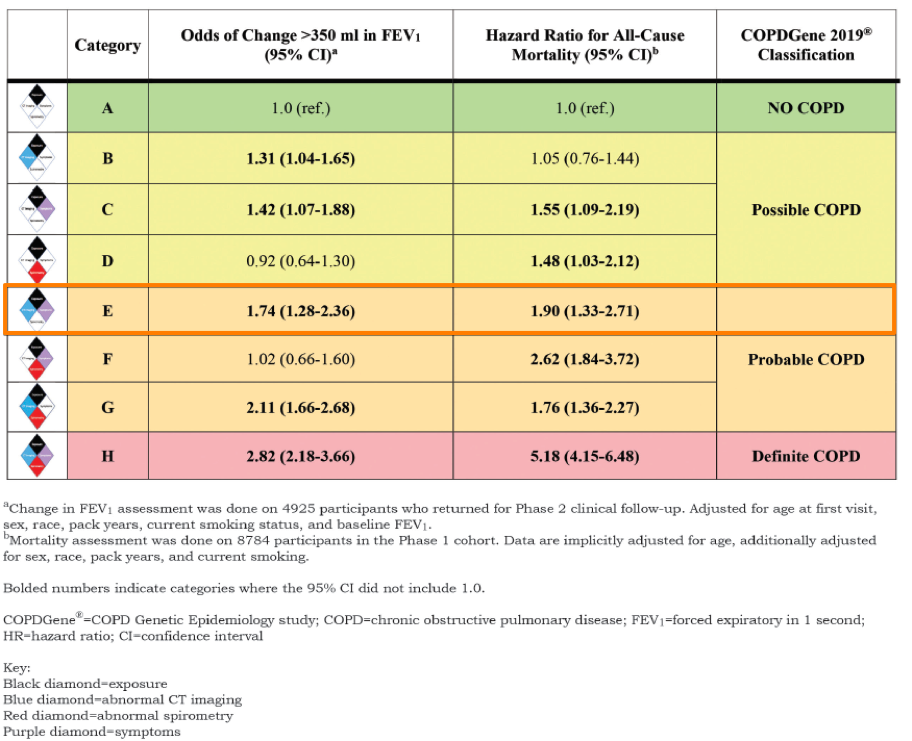
As you can see in the table above, these Category E patients have normal spirometry results, but have a 74% increased risk of FEV1 decline and a 90% increased risk in all-cause mortality.
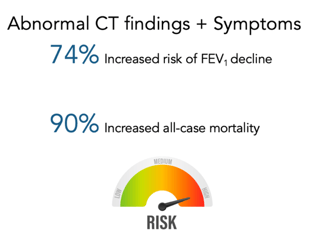
Implications
The authors conclude that their model is able to:
- “Identify significant differences in
physiological, symptomatic and CT structural imaging abnormalities.” - “Characterize patients into mutually exclusive
categories.” Given the heterogeneous
nature of COPD, this is a major advantage over current classification systems. - Consider the “incremental influence of these disease manifestations
on traditional measures of disease progression, including lung function decline
and all-cause mortality.”
“Our comprehensive analyses suggest that spirometry alone fails to capture and contextualize the extent of disease manifestation.”
COPDGene Authors
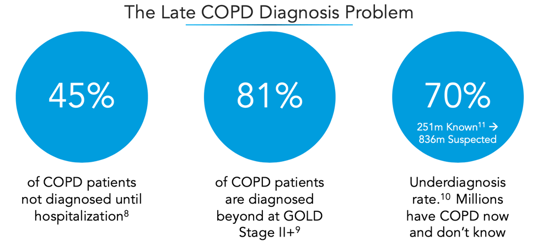
The classification system proposed in the paper would have diagnosed an additional 21.4% of the COPDGene® population with COPD. Since COPD underdiagnosis is such a large problem[6], we have hope that these new criteria will be effective in identifying COPD earlier than it is today.
The potential impact of broad early detection of COPD at the population level is tremendous. Currently, 40-50% of COPD patients are not diagnosed until hospitalization. Quality of life is significantly reduced as disease progresses and cost of care is nearly 10X higher for those with late stage disease vs. early disease[7].
Conclusions
VIDA has long understood the power of CT-based lung imaging analysis, not only for early detection of disease, but also to aid in disease classification, quantitative tracking, treatment selection, and procedural guidance. VIDA and its customers are passionate about advancing the standard of lung care through intelligence. We are delighted to see that industry key opinion leaders have included Quantitative CT (QCT) imaging as a key piece of their newly proposed COPD diagnosis criteria. The new COPDGene classification brings greater sensitivity and precision to providers in an effort to advance the standard of care—a perfect alignment with our mission as a company.
References
[1] “Terminology, Definitions, and Classification of Chronic Pulmonary Emphysema and Related
Conditions,” Thorax, vol. 14, no. 4, pp. 286–299, Dec. 1959.
[2] Committee on Diagnostic Standards for Nontuberculous Respiratory Disease and American Thoracic Society, “Definitions and classification of chronic bronchitis, asthma, and pulmonary emphysema.,” AmRev Respir Dis., vol. 85, pp. 962–769, 1962.
[3] J. Hutchinson, “On the capacity of the lungs, and on the respiratory functions, with a view of establishing a precise
and easy method of detecting disease by the spirometer,” Med Chir Trans, vol. 29, pp. 137–252, 1846.
[4] P. G. Woodruff et al., “Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function,” N.
Engl. J. Med., vol. 374, no. 19, pp. 1811–1821, May 2016, doi: 10.1056/NEJMoa1505971.
[5] E. A. Regan et al., “Clinical and Radiologic Disease in Smokers With Normal Spirometry,” JAMA Intern Med,
vol. 175, no. 9, pp. 1539–1549, Sep. 2015, doi: 10.1001/jamainternmed.2015.2735.
[6] N. Diab et al., “Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease,” Am. J. Respir.
Crit. Care Med., vol. 198, no. 9, pp. 1130–1139, 01 2018, doi:10.1164/rccm.201804-0621CI.
[7] A. J. Guarascio, S. M. Ray, C. K. Finch,and T. H. Self, “The clinical and economic burden of chronic obstructive
pulmonary disease in the USA,” Clinicoecon Outcomes Res, vol. 5, pp. 235–245, Jun. 2013, doi: 10.2147/CEOR.S34321.
[8] A. Khakban et al., “The Projected Epidemic of Chronic Obstructive Pulmonary Disease Hospitalizations over the
Next 15 Years. A Population-based Perspective,” Am J Respir Crit Care Med, vol. 195, no. 3, pp. 287–291, Sep. 2016, doi: 10.1164/rccm.201606-1162PP.
[9] D. W. Mapel, A. A. Dalal, C. M. Blanchette, H. Petersen, and G. T. Ferguson, “Severity of COPD at initial spirometry-confirmed diagnosis: data from medical charts and administrative claims,” Int J Chron Obstruct Pulmon Dis, vol. 6, pp. 573–581, 2011, doi: 10.2147/COPD.S16975.
[10] “Chronic obstructive pulmonary disease (COPD).” [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). [Accessed: 08-Jan-2020].
-end-